Makindo Medical Notes.com |
|
|---|---|
| Download all this content in the Apps now Android App and Apple iPhone/Pad App | |
| MEDICAL DISCLAIMER:The contents are under continuing development and improvements and despite all efforts may contain errors of omission or fact. This is not to be used for the assessment, diagnosis or management of patients. It should not be regarded as medical advice by healthcare workers or laypeople. It is for educational purposes only. Please adhere to your local protocols. Use the BNF for drug information. If you are unwell please seek urgent healthcare advice. If you do not accept this then please do not use the website. Makindo Ltd | |
Cardiac Anatomy and Physiology
-
| About | Anaesthetics and Critical Care | Anatomy | Biochemistry | Cardiology | Clinical Cases | CompSci | Crib | Dermatology | Differentials | Drugs | ENT | Electrocardiogram | Embryology | Emergency Medicine | Endocrinology | Ethics | Foundation Doctors | Gastroenterology | General Information | General Practice | Genetics | Geriatric Medicine | Guidelines | Haematology | Hepatology | Immunology | Infectious Diseases | Infographic | Investigations | Lists | Microbiology | Miscellaneous | Nephrology | Neuroanatomy | Neurology | Nutrition | OSCE | Obstetrics Gynaecology | Oncology | Ophthalmology | Oral Medicine and Dentistry | Paediatrics | Palliative | Pathology | Pharmacology | Physiology | Procedures | Psychiatry | Radiology | Respiratory | Resuscitation | Rheumatology | Statistics and Research | Stroke | Surgery | Toxicology | Trauma and Orthopaedics | Twitter | Urology
Related Subjects: |Cardiac Anatomy and Physiology |Coronary Artery Anatomy and Physiology |Cardiac Electrophysiology |Cardiac Embryology
Anatomy
- Heart is a conical shaped structure with both an apex and base lying in the middle of the mediastinum.
- It is enveloped in a layer of fibrous pericardium. The heart lies sandwiched between the lungs.
- The apex of the cone points down and out and its anatomical surface marking is the mid clavicular line in the 5th Intercostal space. The base of the pyramid faces posteriorly.
- The heart is made up of 4 chambers.
- Two atria which acts as storage vessels for blood returning to the heart
- Two ventricles which act as pumps.
Basic structure
- The left ventricle receives blood from the left atrium across the semilunar mitral valve with its anterior and posterior leaflets and pumps blood into the systemic circulation is larger and has a thick muscular wall.
- The right ventricle receives deoxygenated blood from the vena cava and then the right atrium which enters the right ventricle across the tricuspid valve and then is ejected with systole across the pulmonary valve into the pulmonary artery.
- Borders of the heart.
- Right border of the heart is formed almost entirely by the right atrium.
- Left border of the heart is formed almost entirely by the left ventricle with the left atrial appendage superiorly.
- Base or posterior surface is formed almost entirely by the left atrium which is closely opposed to the oesophagus (important for trans oesophageal echo).
- Inferior or diaphragmatic surface of the heart is made up by the right and left atrium
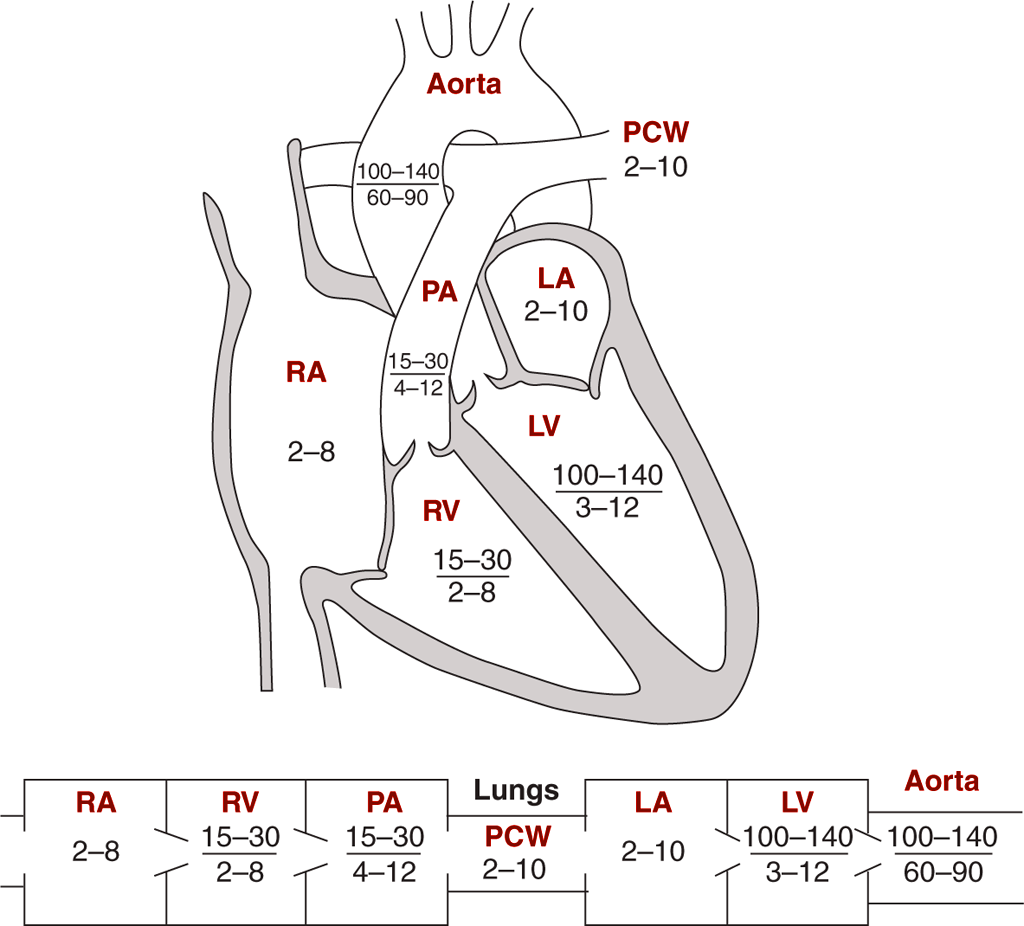
Pressures R v L
Physiology
- Systole is when the ventricle contracts and blood is forced out into the systemic and pulmonary systems.
- Diastole when the ventricles relax and the atria fill. Diastole shortens as heart rate increases
Cardiac output
- Blood volume is 5 L. Volume of LV at the end of diastole = 100 ml.
- Volume in LV at end of systole = 30 ml. Stroke Volume (SV) is 70 ml. Average heart rate 70 bpm.
- Cardiac output = SV (70 ml) x Heart rate (70bpm) = approximately 5L/minute
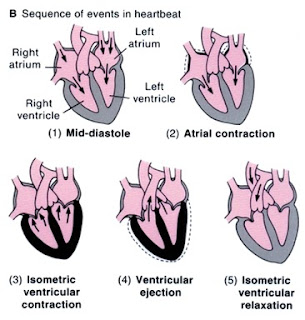 Cardiac cycle
Cardiac cycle
Cardiac cycle
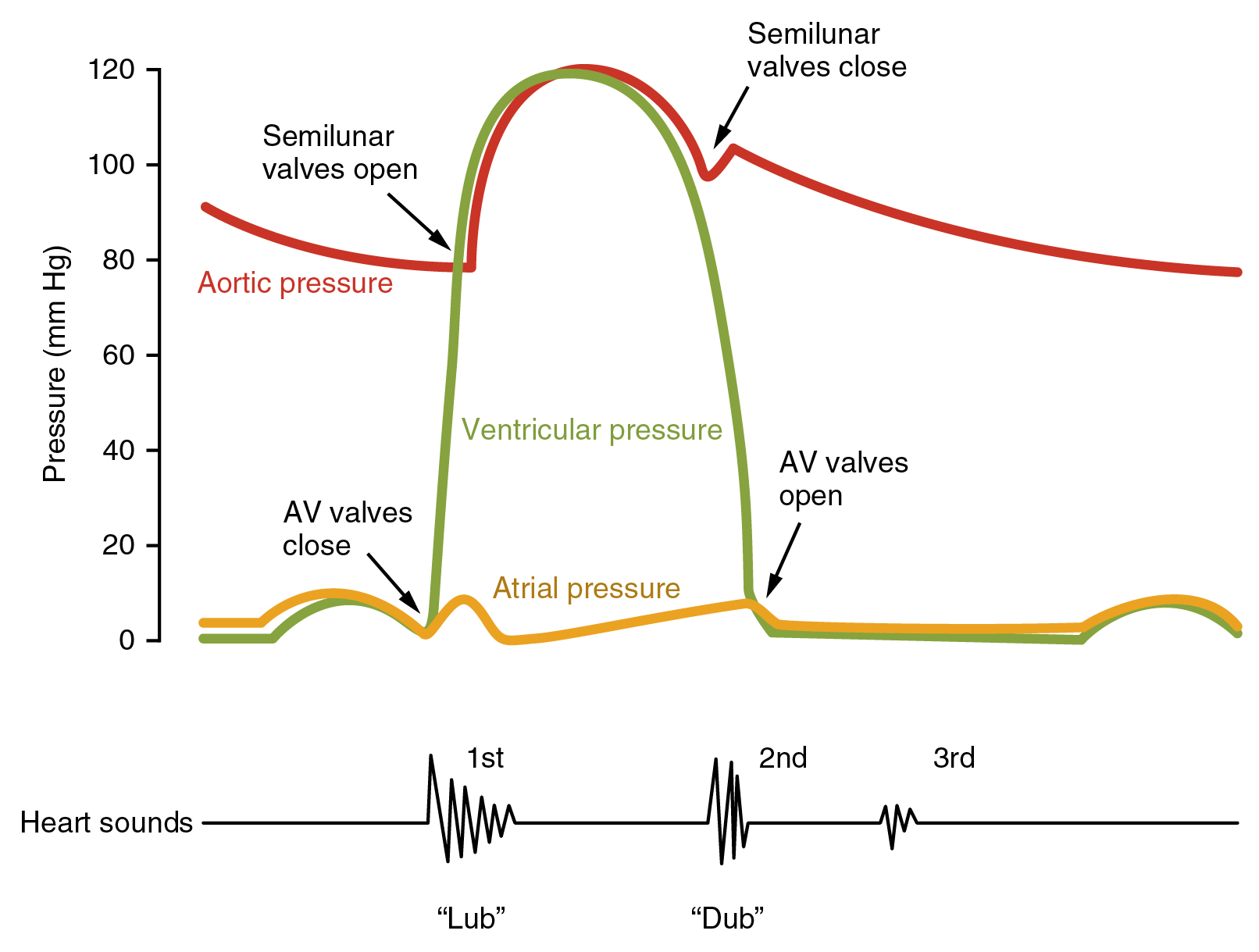
Measuring Cardiac output - Several techniques exist
- Thermodilution technique using a PA catheter - cold dextrose is injected into the Left atrium and the temperature measured using a transducer in the pulmonary artery. The drop in temperature with time infers the volume of blood passing per unit time and so an estimation of cardiac output can be made
- Oesophageal Doppler can visualise and measure using doppler the flow in the descending Aorta. Using a correction factor for the patient's age, weight and height estimation of cardiac output can be made
- Localised alterations to flow depend on localised resistance. Most of this is in arterioles and small arteries.
- Small changes in arterial radius can have large changes on peripheral resistance.
- Capillaries have a much less important role in altering flow patterns.
- During exercise there is an increased heart rate and stroke volume, Renal and gut and skin perfusion falls, Increased muscle and cardiac perfusion
Systemic and pulmonary circulations
- Systems are both in parallel and in series so flow is the same through both.
- Resistance in pulmonary side only 10% of the systemic circulation.
- Systemic pressures 120/80 mmHg. Pulmonary pressures 25/10 mmHg
Coronary anatomy
- The first organ supplied with blood by the heart is the heart.
- Left Mainstem (left coronary artery) arises from the left posterior aortic sinus and branches almost immediately into the left anterior descending (LAD) which travels between right and left ventricles towards the apex. The LAD gives off the diagonal branches (D1, D2) and septal branches
- Circumflex (Cx) which lies in the left AV groove between the left atrium and left ventricle and supplies the vessels of the lateral wall of the left ventricle. 10% of patients have a left dominant circulation in which the Cx also supplies the posterior descending artery (PDA). The Cx also gives off the Marginal branches (M1, M2)
- Right coronary artery is the first branch of the aorta and arises from the anterior aortic sinus and runs in the AV grove between the right atrium and ventricle. It gives off the
- Acute marginal branch runs along the margin of the right ventricle above the diaphragm.
- Sinus node branch in 60% (otherwise supplied by the Cx)
- Atrioventricular node branch
- Posterior descending artery (RCA dominant) in over 65% which supplies the inferior wall of the left ventricle and inferior part of the septum
Cardiac cycle
- The SAN node drives the cardiac cycle. The SAN has an intrinsic rate of 100 bpm but this is slowed by vagal tone to 70 bpm. The SAN pacemaker cell depolarizes spontaneously as it does 70 times per minute and fires of an action potential into the surrounding atrial tissue.
- The myocardial cells depolarize and the action potential spreads like a forest fire across the atrial generating a P wave on the surface ECG. The right atrium depolarizes first - atrial systole.
- Assuming the previous beat was normal then the heart at this point will be near the end of ventricular diastole and the atrial contraction that is caused causes slight additional filling of the ventricles. This is the period of active ventricular filling which is lost with AF.
- Atrial systole adds 15% to ventricular filling. This becomes more important as the heart rate increases and time for diastolic filling decreases.
- Now the depolarization reaches the AV node where there is temporary slowing of passage and this allows the heart maximal time for filling across the mitral and tricuspid valves. This continues as part of the PR interval.
- The depolarization spreads down the bundle of his and into the left and right bundles and Purkinje fibres and spreads quickly across the myocardium. The surface ECG represents ventricular depolarization as a QRS complex.
- As the ventricles contract there is a sudden rise in left ventricular pressures. The mitral and tricuspid valves shut tight (first heart sound) and there is isovolumetric contraction. Eventually, the LV pressures exceed aortic and pulmonary pressures and the aortic and pulmonary valves open and blood is forced into the aorta. This is the ejection phase.
- The ventricle then begins to relax and electrically repolarise. Pressure in the ventricles falls and the aortic and pulmonary valves shut (Second heart sounds). There is isovolumetric relaxation.
- The Mitral and tricuspid valves open and during this period of diastole, there is a period of passive ventricular filling which is usually very rapid.
- The SAN then depolarizes once more
Cellular level
- Pacemaker cells have no resting membrane potential but constantly leak current raising membrane potential rises until it hits the threshold potential. This is due to the influx of calcium. The rate of change of membrane potential can be altered by extrinsic factors thus altering heart rate.
- Myocardial cells exist at a steady membrane potential unless depolarized. Depolarization results in a rapid movement in though fast Na channels of Na+ ions. This also opens calcium channels and calcium enters. This leads to the opening of Ca²⁺ channels in the sarcoplasmic reticulum which causes myocardial contraction.
- Calcium enters through L-type dihydropyridine sensitive channels on the sarcolemma which leads to calcium release from the sarcoplasmic reticulum through RyR2 cardiac ryanodine receptor Excitation contraction coupling
- Myocardial cells surrounded by a membrane (sarcolemma) containing myofibrils surrounded by sarcoplasmic reticulum which form a T system of channels
- The basis of excitation-contraction coupling is the myosin cross bridge. Depolarisation leads to the entry of Ca²⁺ ions into the sarcoplasmic reticulum
- The 100-fold rise in cytosolic calcium saturates all the Ca²⁺ binding sites of troponin C which displaces tropomyosin uncovering myosin-binding sites on actin
- ATPase activity in the myosin head hydrolyses ATP to ADP. There is a conformation change in the relation between actin and myosin which leads to movement or ratcheting such that the actin and myosin filaments slide past each other shortening the sarcomere.
- Ratcheting continues as long as cytosolic Ca²⁺ is elevated but this soon drops as it is actively removed by a sarcoplasmic reticulum ATPase pump. Relaxation follows where troponin C releases calcium and a change in conformation that covers the active binding sites. The sarcomere returns to its initial length.
- Any increase in cytosolic calcium increases the amount of ATP hydrolysed and the force generated. Mechanism includes beta-adrenoreceptor stimulation which increases cAMP which activates protein kinase which increases calcium entry through L-type calcium channels.
- Digoxin increases intracellular calcium levels. Excitation contraction coupling may be impaired in heart failure.
Starling's law
In normal cardiac tissue stretching of the cardiac muscle improves the force and velocity of the corresponding contraction. The stretch of the cardiac muscle is due to filling and therefore equates the volume of the blood in the heart just before ventricular systole. This is the left ventricular end-diastolic volume. This enables the heart to respond to exercise and all manner of increasing cardiac demand. As a heart fails this relationship becomes altered and stretching can then result in no improvement or even a worsening of contractile function.
Exercise
- Physiological changes
- Release of Noradrenaline [US Norepinephrine] and Adrenaline [US Epinephrine] with skeletal muscle vasodilation through beta-2 receptors
- Vasoconstriction to other major organs through alpha-1 mediated receptors
- Increased heart rate and pulse rate and stroke volume through beta-1 receptors
- Bronchodilation through pulmonary beta-2 receptors on bronchial smooth muscles
- Effects
- Heart rate 50/min -> 150/min (x3)
- Stroke volume 80 ml -> 160 ml (x2) per systole
- Peripheral resistance falls
- Cardiac output increases by x 6 times from 4 to 24 l/min and even higher
Cardiac action pacemaker potential at the SA node
Pacemaker cells show a different mechanism of depolarisation than other cells. They are found in the sinoatrial node, atrioventricular node and the Purkinje fibres. The SAN pacemaker cells have the fastest rate of depolarisation.
Ventricular Myocyte Action potential - 5 phases
- Phase 0: Upstroke is due to transient increase in [Na+] conductance and [Na+] movement inwards
- Phase 1: Brief repolarization due to increased [K+] outwards
- Phase 2: Plateau due to increase [K+] out and [Ca2+] in through L-type calcium channels
- Phase 3: Repolarization as [Ca2+] conductance decreases, and there is a large [K+] movement out leading to hyperpolarisation of the cell
- Phase 4: Return to resting potential near the K+ equilibrium potential
Pacemaker cell at SA Node - have only 3 phases
- Phase 0: Upstroke or depolarisation is primarily due to increased Ca2+ conductance and Calcium entry through both Transient and long-acting Calcium channels.
- Phase 3: Repolarization occurs K+ conductance increases and Ca2+ conductance decreases
- Phase 4: Resting membrane potential returns as the inward potassium channels close and again as the cell hyperpolarises there is a small [Na+] influx through the "funny" channels. The so-called Funny Sodium If current and background inward sodium channels are open too - If. What's so funny? Well, it is so-called as it opens when the cell is hyperpolarised. Most normal channels open when the cell is depolarized. As they are open the cell slowly depolarises.